|
Case Report
Aspergillus brain abscess presents as sinusitis in immunosuppressed and hyperglycemic patient
1 Medical Student at Medical College of Wisconsin, Milwaukee, Wisconsin, USA
2 Graduate of Drake University, Biochemistry, Cell & Molecular Biology, Chemistry, Des Moines, Iowa, USA
3 Associate Professor, Internal Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
4 Assistant Professor, Internal Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
Address correspondence to:
Sarah C Kurkowski
4359 S 110th Street, Greenfield, WI 53228,
USA
Message to Corresponding Author
Article ID: 100069Z09SK2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Kurkowski SC, Thimmesch MJ, Jha P, Abdelgadir YH. Aspergillus brain abscess presents as sinusitis in immunosuppressed and hyperglycemic patient. J Case Rep Images Med 2022;8(1):13–18.ABSTRACT
Introduction: There are currently very few documented case reports of Aspergillus brain abscesses in the setting of persistent sinusitis symptoms and comorbid conditions of Type II Diabetes Mellitus and steroid treatment. Many cases of intracranial aspergillosis are secondary to a disseminated invasive Aspergillus infection or primary pulmonary aspergillosis. This case illustrates the importance and vitality of considering the rare but potentially lethal diagnosis of Aspergillus fumigatus brain abscess in the differential of persistent sinusitis, even in the absence of systemic symptoms.
Case Report: A 73-year-old female with risk factors of Type II Diabetes Mellitus (T2DM) and steroid treatment presented to the hospital with persistent sinusitis symptoms lasting three months. She subsequently was diagnosed with Aspergillus brain abscess after bicoronal bifrontal craniotomy with pericranial flap. The patient was treated with voriconazole and amphotericin B for 3–6 months post-resection and follow-up with neurosurgery to monitor abscess reduction. She was admitted five more times after initial diagnosis for sequelae related to the brain abscess. The initial abscess did decrease in size but then began to worsen. The patient unfortunately passed away six months after initial presentation and diagnosis.
Conclusion: Few cases of invasive rhino-orbito-cerebral Aspergillus brain abscesses secondary to Aspergillus sinus infections have been documented. If missed it can be lethal. Therefore, when a patient presents with persistent sinusitis, in the relevant context, Aspergillus brain abscesses are an important differential diagnosis that warrant consideration.
Keywords: Aspergillus brain abscess, Aspergillus fumigatus, Aspergillus sinusitis, Immunocompromised
Introduction
Aspergillus, a moniliaceous mold found in soil and decaying vegetation is an opportunistic fungus that demonstrates septate hyphae with acute angle branching, which often take advantage of immunocompromised states [1],[2]. Neurologic infectivity is majorly affected by immune status of the patient and virulence of the specific strain of the culprit fungus. Fungal neuroinfections cause significant morbidity. In a meta-analysis reviewing 135 articles from 2000 to 2018, the main presentations of fungal neuroinfections documented were meningitis, encephalitis, hydrocephalus, cerebral abscesses, and stroke symptoms [2].
The most common causes of fungal brain abscesses in immunocompromised patients are Candida, Aspergillus, Cryptococcus, and Mucor. A. fumigatus, Aspergillus flavus, Aspergillus amstelodami, Aspergillus sjdowi, Aspergillus candidus are the subspecies of Aspergillus that have been shown to be most likely to cause central nervous system (CNS) infections. Of these subtypes, A. fumigatus is most often the culprit [3]. A brain abscess due to Aspergillus can be due to direct or hematogenous spread from a local or distant site of infection. Direct extension is often through the paranasal sinuses, ear, nose, or eye [1]. Aspergillus spores have a preference for the anterior and middle cranial fossa [4]. In this case specifically, a frontal and ethmoidal sinus Aspergillus infection likely led to direct invasion of the frontal lobes.
The patient presented here had a significant immunocompromised state due to uncontrolled Type II Diabetes Mellitus and steroid treatment, putting the patient at increased risk for opportunistic infections such as Aspergillus. Diagnosis of Aspergillus brain abscess, although rare, should always be included on differential when evaluating hyperglycemic and immunosuppressed patients with sinusitis symptoms. Aspergillus brain abscesses are severe and can progress to frontal bone erosion (or the cranial bone closest to the abscess), subsequent neurological sequelae, and therefore should not be overlooked or disregarded from the differential. Our objective is to highlight the importance of including Aspergillus brain abscesses in the differential diagnosis in individuals presenting with sinusitis symptoms whose medical history showcases immunosuppression and hyperglycemia.
Case Report
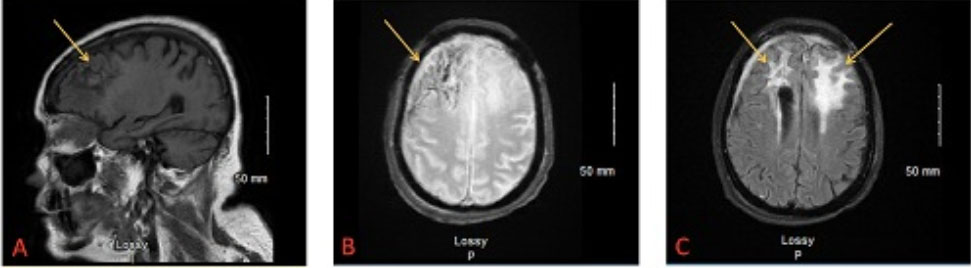
A 73-year-old female with a past medical history of poorly controlled Type II Diabetes Mellitus and arthritis treated with steroids presented to the emergency department with sinusitis symptoms. Three months prior to presentation, she developed a copious, thick, yellow-green nasal discharge, and headaches. She traveled from Mexico to Milwaukee since these symptoms persisted, and upon arrival to the emergency department was admitted. Computed tomography (CT) head scan without contrast showed a likely 5.7 cm brain abscess located in the anterior cranial fossa associated with bilateral frontal bones and ethmoid plate; imaging also demonstrated opacification of both frontal sinuses (Figure 1A, Figure 1B, Figure 1C). To remove the abscess, a bicoronal bifrontal craniotomy with pericranial flap was performed by neurosurgery. CALCOs (Calcofluor White fungal stain) from the surgical abscess were 1/4 positive for septate hyphae concerning Aspergillus; fungal cultures of resected tissue showed A. fumigatus. Blood cultures were negative for fungi. The patient was treated with antifungals—amphotericin and voriconazole—which she would continue for 3–6 months post-surgery.
Subsequently, over the following five months, the patient returned to the emergency department on five separate occasions. Most of these hospitalizations were secondary to the brain abscess. She developed seizures confirmed by electroencephalogram (EEG), subdural hematoma, and right eye blindness.
Two and a half months post-op, outpatient magnetic resonance imaging (MRI) showed marked reduction in frontal lobe abscess, approximately 2.2×2.5×4.2 cm residual intraparenchymal abscess compared to 5.7 cm diameter abscess at presentation. Also appreciated was a reduction in midline shift, but new extension of right ethmoid sinus infection into medial right orbit (Figure 1D and Figure 1E).
The last hospitalization (five months post-craniotomy/initial hospital admission), the patient was admitted for right eye blindness. This blindness had persisted for three days prior to the patient arriving at the hospital. At that time, the patient underwent endoscopic sinus surgery and right orbitotomy. Cultures from the right orbit showed A. fumigatus, while blood cultures were still negative.
One month after being discharged for right eye blindness (six months after initial diagnosis), the patient followed up outpatient for a subsequent postoperative MRI. This imaging showed overall stable postoperative findings, however noted improvement in some regions and worsening in others. Her right frontal lobe abscess, first noted on CT six months prior, and paranasal sinus infection had improved dramatically. Nodular enhancement in the periphery of the left frontal lobe still persisted while left frontal lobe vasogenic edema had increased. In addition, there was notable dural and subdural thickening over the right frontal convexity. This increased concern for progression of the abscess (Figure 2A, Figure 2B, Figure 2C).
Unfortunately, one week after the above findings were noted on MRI, the patient passed away. The total time from initial presentation and diagnosis to death was approximately six months.
Discussion
Here we report a case of a bilateral frontal brain abscess due to A. fumigatus. The abscess likely developed due to the longstanding history of sinusitis and immunosuppression from steroid use and poorly controlled Type II Diabetes Mellitus. The patient’s presentation first was thought to be solely due to sinusitis (three months of nasal drainage and headache), however, subsequent imaging elucidated brain involvement. Aspergillus fumigatus brain abscesses that present with symptoms of sinusitis have been characterized as an easily missed diagnosis [5]. Cerebral abscesses secondary to Aspergillus sinusitis may be rare but are often lethal to patients [6].
It is often assumed that an Aspergillus brain abscess represents underlying disseminated invasive Aspergillus infection [1]. For example, case reports have been published with immunocompromised patients developing an intracranial Aspergillus abscess secondary to a pulmonary Aspergillus abscess [3],[7]. Additionally, other documented cases describe intracranial aspergillosis in organ transplant patients and intensive care unit (ICU) patients who were hospitalized prior to developing the infection [8],[9].
Multiple studies have listed the most common presentations of intracranial aspergillosis as mental status changes, seizures, focal neurological deficits, sudden headache, loss of consciousness, proptosis, vision changes, nasal discharge, cranial nerve palsies, etc. [9],[10],[11],[12]. One study reported altered mental statuses as accounting for the largest percentage of presentations [9]. Our patient’s presentation was much more insidious and could have been easily missed and passed off as just sinusitis.
However, our patient did not have disseminated or pulmonary aspergillosis at presentation or when an abscess was found on imaging. She also was not hospitalized prior to the development of her symptoms, and her presenting symptom of sinusitis was not among the most common presentations of an Aspergillus brain abscess.
As our patient’s CT scan showed, an infection in the frontal/ethmoidal sinuses can spread to the frontal lobes, either by direct or hematogenous spread. Brain abscesses due to Aspergillus are rare and an example of an opportunistic infection. This cause should be included in the differential diagnoses in patients presenting with longstanding severe sinusitis symptoms, especially in those with current or past medical history of immunosuppression (which is a well-known risk factor for opportunistic infections). It has also been reported that patients with hyperglycemia (such as from poorly controlled diabetes) show increased rates of mortality due to brain abscesses [13]. This highlights another important reason to keep Aspergillus brain abscesses on the differential in a patient with a history of poorly controlled Type II Diabetes Mellitus, as seen here. This case demonstrates a unique intertwining of the risk factors of immunosuppression and hyperglycemia in the development of an Aspergillus brain abscess, and the importance of not ruling out potential diagnoses simply because of their rarity.
One article listed vulnerable individuals for CNS fungal infections as those who had hematological malignancies, transplant recipients, and HIV [14]. However, our patient did not have any of these diagnoses. It is important to mention that our patient clearly had a predisposition to opportunistic infections due to steroid treatment and uncontrolled T2DM.
As done in our patient, the pillar of cerebral aspergillosis treatment is surgical resection followed by systemic antifungal therapy [15]. Sole antifungal therapy was shown to have over 90% mortality rate in CNS aspergillosis, but surgical resection reduced the rate from 64% to 39%. The combination of both decreases mortality rate further, particularly when the orbit is involved in addition to brain parenchyma [10],[16]. After surgical resection of abscess, a combination of voriconazole and amphotericin B was used to treat this patient. Voriconazole is a moderately lipophilic compound with good CNS penetration, making it a well-established first-line treatment for CNS aspergillosis. Another well-identified treatment for fungal neuroinfections is the use of amphotericin B and flucytosine for cryptococcal meningitis [2]. Prior to voriconazole, amphotericin B was the mainstay for cerebral aspergillosis. In a randomized controlled study, voriconazole was compared to amphotericin B for invasive aspergillosis. Voriconazole was found to be better than amphotericin B, specifically having a 21.2% greater response than amphotericin B. This study also found that voriconazole led to greater survival rates. Voriconazole reaches concentrations in the brain parenchyma that are effective for treatment of CNS aspergillosis [17]. Within fungal abscesses in the brain parenchyma, voriconazole has been found to penetrate both brain tissue and the abscess material itself. Its peak concentration within the brain is higher than that achieved within the plasma [18].
However, there have been cases documented of failed voriconazole treatment in the instance of invasive CNS aspergillosis. In a previous case report, a patient on corticosteroid and immunomodulator therapy was diagnosed with invasive cerebral aspergillosis and subsequently failed treatment with voriconazole alone. However, when treated with a combination of voriconazole and amphotericin B, the patient recovered well [19]. The concern arose that A. fumigatus exhibits increasing rates of azole resistance. Recently, Dutch guidelines for antibiotic use recommended that a combination of voriconazole and liposomal amphotericin B or echinocandin should be used to treat A. fumigatus (at this time, 12.9% of A. fumigatus were azole-resistant) until strain sensitivity resulted [17]. Therefore, as it was determined that the tissue sample from our patient’s resection was positive for A. fumigatus, treatment with voriconazole and amphotericin B was chosen.
Following initial imaging with CT, MRI was chosen for the rest of the patient’s imaging. Magnetic resonance imaging (MRI) seems to be a better tool for diagnostic purposes and involvement of important neural parenchymal structures. One article outlined specific MRI characteristics that should heighten suspicion for an intracranial fungal granuloma: “faintly enhancing intracranial masses, poorly defined margins, central hypodensities, and disproportionate cerebral edema” [12].
An interesting mechanism of Aspergillus extension into the brain was proposed as being through a perineural pathway by Safdar et al. where Aspergillus may travel via nerves from sinuses to the brain to establish intracranial infection [20]. It is well-established that viral respiratory infection can be complicated by superimposed bacterial pneumonia. Our patient presented here did not have pneumonia, but did have three months of persistent sinusitis. It is reasonable to wonder if she had a primary viral sinus infection that led to the development of a secondary fungal infection (such as Aspergillus). Her assumed Aspergillus infection of the frontal and ethmoid sinuses allowed for erosion of bone and infiltration into brain parenchyma. Potentially, her sinusitis began as viral in nature and Aspergillus was a superimposed fungal infection, to which she was susceptible due to her immunocompromised state. A very recent article, “Invasive Aspergillosis of nose and paranasal sinus in COVID-19 convalescents: Mold goes viral?,” was published in January 2022 on post-COVID fungal infections of the nose and paranasal sinuses. The study stated that during the first wave of COVID-19, most post-COVID fungal infections seemed to be due to mucormycosis, however, invasive Aspergillus infections seemed to be rising in number during the second wave of COVID-19 [21]. Our patient tested negative for COVID-19 at presentation to the emergency department. However, this does not rule out a previously resolved COVID-19 infection that could have predisposed our patient to an invasive Aspergillus sinus infection. The previously mentioned article stated that 46% of their patients who had COVID-19 infection followed by an invasive fungal infection were young and did not have predisposing comorbidities. Compared to their study’s patients, our patient did have underlying comorbidities (steroid treatment and uncontrolled T2DM) and therefore was significantly more predisposed to develop an invasive Aspergillus sinus infection after infection with COVID-19. COVID-19 virus is known to decrease CD8+ and CD4+ T cells which would further increase the susceptibility of post-COVID patients to developing fungal infections [21]. In addition, glucocorticoid treatment (our patient was taking for arthritis) inhibits inflammatory molecules like adhesion molecules, cytokines, and chemokines that aid in the immune system’s response to pathogens [22]. Shetty et al. also made clear that there are few case reports of rhino-orbito-cerebral aspergillosis compared to the many documented cases of rhino-orbito-cerebral mucormycosis. Two of these cases did involve the primary site of infection being the ethmoid sinuses and subsequent extension of infection to frontal lobes, similar to our patient [23],[24]. One case postulated that primary sinus Aspergillus infection could be a chronic infective process, similar to the chronicity of aspergilloma of the lung [24]. This further validates the importance of our patient’s case as it is one of few on invasive rhino-orbitocerebral Aspergillus abscesses presumably secondary to Aspergillus sinus infection.
Conclusion
Diagnosis of Aspergillus brain abscess should always be included on differential when evaluating hyperglycemic and immunosuppressed patients with sinusitis symptoms. Aspergillus brain abscesses are severe and can progress to frontal bone erosion (or cranial bone closest to abscess) and significant neurologic sequelae (seizures, right eye blindness, etc.). This diagnosis has a high mortality rate, poor prognosis, and is an easily missed diagnosis when the presenting symptom is persistent sinusitis. Proper treatment with surgical resection of abscess and subsequent combination therapy with voriconazole and amphotericin B can increase the likelihood of survival, but does not eliminate the significant risk of patient death, as in our patient. It is plausible that previous and resolved COVID-19 infection (not confirmed in our patient) could predispose to fungal sinus infections, A. fumigatus in particular. Few cases of invasive rhino-orbito-cerebral Aspergillus brain abscesses secondary to Aspergillus sinus infections have been documented. If missed it can be lethal. Therefore, when a patient presents with persistent sinusitis, in the relevant context, Aspergillus brain abscesses are an important differential diagnosis that warrant consideration.
REFERENCES
1.
Chakrabarti A. Epidemiology of central nervous system mycoses. Neurol India 2007;55(3):191–7. [CrossRef]
[Pubmed]

2.
Góralska K, Blaszkowska J, Dzikowiec M. Neuroinfections caused by fungi. Infection 2018;46(4):443–59. [CrossRef]
[Pubmed]

3.
Artico M, Pastore FS, Polosa M, Sherkat S, Neroni M. Intracerebral Aspergillus abscess: Case report and review of the literature. Neurosurg Rev 1997;20(2):135–8. [CrossRef]
[Pubmed]

4.
Chen S, Pu JL, Yu J, Zhang JM. Multiple Aspergillus cerebellar abscesses in a middle-aged female: Case report and literature review. Int J Med Sci 2011;8(7):635–9. [CrossRef]
[Pubmed]

5.
Shuper A, Levitsky HI, Cornblath DR. Early invasive CNS aspergillosis. An easily missed diagnosis. Neuroradiology 1991;33(2):183–5. [CrossRef]
[Pubmed]

6.
Patron V, Orsel S, Caire F, Turlure P, Bessède JP, Aubry K. Endonasal trans-ethmoidal drainage of a cerebral abscess. Skull Base 2010;20(5):389–92. [CrossRef]
[Pubmed]

7.
Sugawara A, Ebina K, Hirano T, Ohi H, Yoshimura S, Akabane M. Multiple aspergillus brain abscess complicated with systemic lupus erythematosus—Case report. [Article in Japanese]. Neurol Med Chir (Tokyo) 1991;31(13):986–90. [CrossRef]
[Pubmed]

8.
Kim MJ, Kim MK, Kang CK, et al. A case of acute cerebral aspergillosis complicating influenza A/H1N1pdm 2009. Infect Chemother 2013;45(2):225– 9. [CrossRef]
[Pubmed]

9.
Torre-Cisneros J, Lopez OL, Kusne S, et al. CNS aspergillosis in organ transplantation: A clinicopathological study. J Neurol Neurosurg Psychiatry 1993;56(2):188–93. [CrossRef]
[Pubmed]

10.
Al-Maskari N, Hussain I, Jumaa S, Al-Shail EA. Aspergillus flavus-induced brain abscess in an immunocompetent child: Case report. Sultan Qaboos Univ Med J 2016;16(2):e246–9. [CrossRef]
[Pubmed]

11.
Lee GJ, Jung TY, Choi SM, Jung MY. Cerebral aspergillosis with multiple enhancing nodules in the right cerebral hemisphere in the immune-competent patient. J Korean Neurosurg Soc 2013;53(5):312–5. [CrossRef]
[Pubmed]

12.
Mohindra S, Mukherjee KK, Chhabra R, Gupta SK, Gupta R, Khosla VK. Invasive intracranial aspergillosis: The management dilemmas. Surg Neurol 2008;69(5):496–505. [CrossRef]
[Pubmed]

13.
Zhang F, Hsu G, Das S, Chen Y, August M. Independent risk factors associated with higher mortality rates and recurrence of brain abscesses from head and neck sources. Oral Surg Oral Med Oral Pathol Oral Radiol 2021;131(2):173–9. [CrossRef]
[Pubmed]

14.
Black KE, Baden LR. Fungal infections of the CNS: Treatment strategies for the immunocompromised patient. CNS Drugs 2007;21(4):293–318. [CrossRef]
[Pubmed]

15.
Shamim MS, Enam SA, Ali R, Anwar S. Craniocerebral aspergillosis: A review of advances in diagnosis and management. J Pak Med Assoc 2010;60(7):573–9.
[Pubmed]

16.
Mody KH, Ali MJ, Vemuganti GK, Nalamada S, Naik MN, Honavar SG. Orbital aspergillosis in immunocompetent patients. Br J Ophthalmol 2014;98(10):1379–84. [CrossRef]
[Pubmed]

17.
von Lilienfeld-Toal M, Wagener J, Einsele H, Cornely OA, Kurzai O. Invasive fungal infection. Dtsch Arztebl Int 2019;116(16):271–8. [CrossRef]
[Pubmed]

18.
Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clin Microbiol Rev 2014;27(1):68–88. [CrossRef]
[Pubmed]

19.
Ehrmann S, Bastides F, Gissot V, et al. Cerebral aspergillosis in the critically ill: Two cases of successful medical treatment. Intensive Care Med 2005;31(5):738–42. [CrossRef]
[Pubmed]

20.
Safdar A, Dommers MP Jr, Talwani R, Thompson CR. Intracranial perineural extension of invasive mycosis: A novel mechanism of disease propagation by Aspergillus fumigatus. Clin Infect Dis 2002;35(5):e50–3. [CrossRef]
[Pubmed]

21.
Shetty S, Shilpa C, Kavya S, Sundararaman A, Hegde K, Madhan S. Invasive Aspergillosis of nose and paranasal sinus in COVID-19 convalescents: Mold goes viral? Indian J Otolaryngol Head Neck Surg 2022;1–6. [CrossRef]
[Pubmed]

22.
van der Velden VH. Glucocorticoids: Mechanisms of action and anti-inflammatory potential in asthma. Mediators Inflamm 1998;7(4):229–37. [CrossRef]
[Pubmed]

23.
Kawakami N, Nishizaki T, Sugiyama S, Ito H. Aspergillus brain abscess in a patient with normal immunity—Case report. Neurol Med Chir (Tokyo) 1994;34(4):237–40. [CrossRef]
[Pubmed]

24.
Lowe J, Bradley J. Cerebral and orbital Aspergillus infection due to invasive aspergillosis of ethmoid sinus. J Clin Pathol 1986;39(7):774–8. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Sarah C Kurkowski - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Michael J Thimmesch - Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Pinky Jha - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Yasir H Abdelgadir - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Sarah C Kurkowski et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.