|
Case Report
Severe COVID-19 in a kidney transplant recipient with acquired hypogammaglobulinemia: A case report
1 Medical Student, Department of Clinical Medicine, University of Bergen, Bergen, Norway
2 Nephrologist and PhD candidate, Department of Clinical Medicine, University of Bergen, Bergen, Norway; Department of Medicine, Haukeland University Hospital, Bergen, Norway
3 Anesthesiologist and Professor, Department of Clinical Medicine, University of Bergen, Bergen, Norway; Department of Anesthesiology & Intensive Care Medicine, Haukeland University Hospital, Bergen, Norway
4 Nephrologist and PhD, Department of Transplantation Medicine, Oslo University Hospital – Rikshospitalet, Oslo, Norway
5 Nephrologist and Associate Professor, Department of Clinical Medicine, University of Bergen, Bergen, Norway; Department of Medicine, Haukeland University Hospital, Bergen, Norway
Address correspondence to:
Mariell Rivedal
Department of Clinical Medicine, University of Bergen, Jonas Lies vei 91B, Bergen, Vestland 5021,
Norway
Message to Corresponding Author
Article ID: 100070Z09MR2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Rivedal M, Haaskjold YL, Guttormsen AB, Midtvedt K, Knoop T. Severe COVID-19 in a kidney transplant recipient with acquired hypogammaglobulinemia: A case report. J Case Rep Images Med 2022;8(2):1–6.ABSTRACT
Introduction: Kidney transplant recipients have an increased risk of a severe clinical course and mortality due to coronavirus disease 2019 compared to that in the average population, and their treatment options are limited because reduced immunosuppression may lead to graft rejection. Herein, we describe a successful therapeutic regime in a kidney transplant recipient who suffered from coronavirus disease 2019-associated acute respiratory distress syndrome.
Case Report: In this case report, we describe the course and management of a kidney transplant recipient who had severely reduced graft function (estimated glomerular filtration rate: 10–14 mL/min/1.73 m2) and acquired hypogammaglobulinemia and was consequently hospitalized and treated for severe coronavirus disease 2019. She presented with gastrointestinal symptoms, followed by increasing dyspnea, which rapidly progressed to acute respiratory distress syndrome. During hospitalization, she was treated under a ventilator (prone positioning) and with convalescent plasma, dexamethasone, careful monitoring of immunosuppression, continuous venovenous hemofiltration, and venovenous extracorporeal membrane oxygenation. Owing to successful treatment, the patient was discharged from the hospital after 74 days in a good condition and with a well-functioning kidney graft.
Conclusion: Convalescent plasma, dexamethasone, monitoring of immunosuppression, continuous venovenous hemofiltration, and venovenous extracorporeal membrane oxygenation might be effective therapeutic options in kidney transplant recipients and other immunosuppressed patients with coronavirus disease 2019-associated acute respiratory distress syndrome.
Keywords: Acute respiratory distress syndrome, COVID-19, Immunosuppression therapy, Kidney transplant recipient
Introduction
In kidney transplant recipients, the risk of a severe clinical course and mortality due to coronavirus disease 2019 (COVID-19) is higher than that in the average population [1]. This risk is due to the use of immunosuppressants, underlying chronic kidney disease, and associated comorbidities [2]. Treatment options have been limited because reduced immunosuppression may lead to graft rejection, that is, reduced kidney function, which is known to be a risk factor for an impaired outcome. Thus, it is important to investigate which therapies are effective in kidney transplant recipients, and other immunosuppressed patients, with COVID-19.
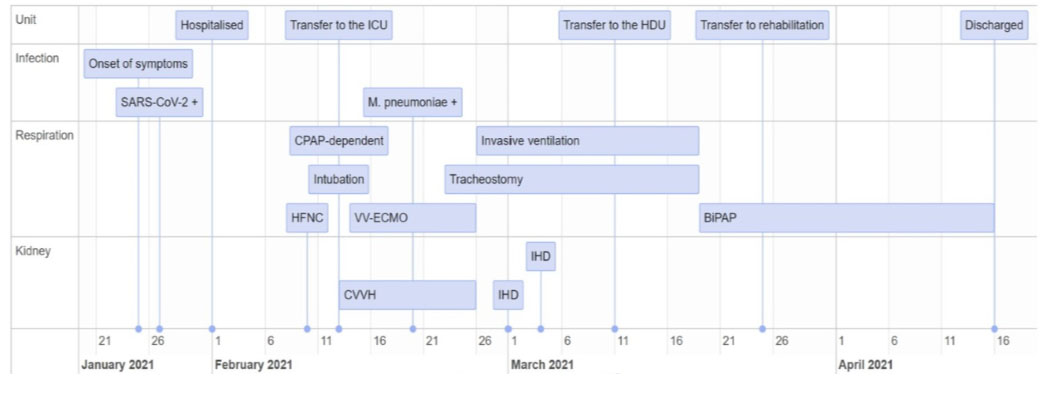
We describe the course of an immunodeficient kidney transplant recipient with COVID-19-associated acute respiratory distress syndrome (ARDS) and multiorgan failure. Important events are shown in Figure 1. The patient was successfully treated with dexamethasone, convalescent plasma, continuous venovenous hemofiltration (CVVH), and venovenous extracorporeal membrane oxygenation (VV-ECMO).
Case Report
A 44-year-old woman who underwent kidney transplantation in 2001 due to crescentic glomerulonephritis, caused by granulomatosis with polyangiitis (GPA), was hospitalized for COVID-19. On admission, the regimen for maintenance immunosuppression included prednisone (5 mg once daily) and tacrolimus (3 mg twice daily). Due to intolerance of mycophenolate mofetil, rituximab (1000 mg) was administered twice yearly for GPA during 2012–2019 but was withdrawn due to recurrent infections. Regular immunoglobulin replacement therapy [intravenous immunoglobulin (IVIG)] every four weeks was initiated from 2020 because of acquired hypogammaglobulinemia due to the rituximab therapy. A gradual decline in graft function [estimated glomerular filtration rate (eGFR), generally <15 mL/min/1.73 m2, calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation] also led to a medical workup and pre-dialytic waiting for re-transplantation.
On January 27, 2021, the patient had suffered from a sore throat and low-grade fever for one week and thus performed a reverse transcription-polymerase chain reaction assay for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), revealing a positive result. Her general condition deteriorated, and on admission, on February 1 (day 0), she had nausea, abdominal pain, and fever (39°C). Laboratory tests revealed the following findings: C-reactive protein (CRP), 49 mg/L (<5); leukocytes, 5.7×109/L (3.5–11.0); neutrophils, 5.1×109/L (1.7–8.2); creatinine, 4.81 mg/dL; and eGFR, 10 mg/mL/1.73 m2. (Arterial blood gas values are shown in Table 1, and other laboratory values are shown in Figure 2). She displayed signs of mild respiratory distress, with a respiratory rate of 18 breaths/min and a SpO2 of 96% at ambient air. Her physical examination results were normal. In accordance with the Norwegian COVID-19 immunization program, as of January 2021, she had not been vaccinated against COVID-19. On admission, low-molecular-weight heparin treatment was initiated.
The patient remained stable during the next five days, following which her SpO2 decreased to 92% at ambient air. She developed diarrhea, emesis, and increased abdominal pain. Chest radiography revealed a newly acquired lobar consolidation in the right lung (Figure 3A). Subsequently, her CRP and creatinine levels increased, initiating rapid clinical and biochemical deterioration, with increasing dyspnea and nausea. Chest radiography revealed increased consolidation and pulmonary edema. Treatment with dexamethasone (6 mg once daily) and convalescent plasma (300 mL/unit, administered on days 10, 11, 17, and 18) was started. Initially, the tacrolimus dose remained unchanged. High-flow nasal cannula oxygen therapy was initiated, but three days later, the patient became entirely dependent on continuous positive airway pressure, requiring higher positive end-expiratory pressure and increased supplemental oxygen.
Owing to her unstable condition, she was urgently transferred to the intensive care unit on day 12. Decreased oxygenation, characterized by SpO2 70%, pO2/FiO2 9.4 kPa, and severe exhaustion, led to immediate intubation, complicated by infraglottic granulomas from her GPA, and ventilation in a prone position. To ease the immunosuppressive load, tacrolimus administration was paused at the time of intubation, although dexamethasone treatment was continued. Intravenous immunoglobulin was administered the following day. Continuous venovenous hemofiltration was initiated due to increasing edema and kidney failure. Chest radiograph revealed distinct bilateral progression of the infiltrates (Figure 3B), and the pO2/FiO ratio decreased to 6.3 kPa. Consequently, VV-ECMO was established on day 13.
The following day, the patient’s condition remained unchanged. She was diagnosed with Mycoplasma pneumoniae infection. Treatment with azithromycin, meropenem, and prophylactic trimethoprim/sulfamethoxazole against Pneumocystis jirovecii was initiated. Gradually, the pO2/FiO2 ratio improved, and chest radiograph showed fewer infiltrates (Figure 3C). Percutaneous dilation tracheostomy was performed on day 22. After two weeks, she was weaned from VV-ECMO, and intermittent hemodialysis (IHD) was initiated. Low-grade tacrolimus treatment was initiated to protect the function of the remaining kidney graft. She received IHD on the days 3 and 6 after CVVH termination. Subsequently, her graft function recovered, making her independent of dialysis during the rest of her hospital stay. Simultaneously, she experienced respiratory improvement (Figure 3D), and on day 38, she was transferred to a high-dependency unit for weaning from ventilation and intensified physical therapy.
On day 52, she was transferred to a post-COVID-19 rehabilitation center and was finally discharged on April 16, which was 74 days after initial hospitalization. Neither SARS-CoV-2 spike nor nucleocapsid immunoglobulin (Ig) G was detected at discharge. The patient’s graft function returned to its habitual levels (eGFR 10–14 mL/min/1.73 m2). Three months after discharge, her spirometry results were similar to her pre-COVID-19 levels [forced vital capacity (FVC), 2.88 (75%); forced expiratory volume in 1 second (FEV1), 2.35 (76%); FEV1/FVC, 0.82], revealing a restrictive pattern and lower gas diffusion. Four months later, spirometry results revealed further improvement, and the patient was again waitlisted for re-transplantation. The patient was vaccinated after discharge (Comirnaty, BNT162b2; Pfizer/BioNTech), following which she had COVID-19-like illness, but thus far, there has been no SARS-CoV-2 detectable spike IgG.
Discussion
This report presents the case of a kidney transplant recipient with COVID-19-associated ARDS and multiorgan failure, which highlights the importance of identifying effective therapies for vulnerable patients.
Several case reports describe kidney transplant recipients with COVID-19, although with variable severity and outcome. The majority describe a clinical course without the need of CVVH or VV-ECMO treatment [3],[4],[5],[6],[7],[8]. Furthermore, to the best of our knowledge, most case reports describing the course of COVID-19-associated ARDS and multiorgan failure in kidney transplant recipients either fail to report the long-term outcome [9] or report unsuccessful outcomes [10],[11], most likely due to comorbidities and late diagnosis. What makes this case report unique is that it does not only describe a successful course of COVID-19-associated ARDS and multiorgan failure in a kidney transplant recipient; the patient also experienced complete renal and pulmonary recovery, despite several comorbidities and being especially vulnerable to infection in general.
The COVID-19 course was complicated by the patient’s GPA, hypogammaglobulinemia, and kidney transplant status. Kidney transplant recipients with COVID-19 need renal replacement therapy more often than nontransplant patients [1]. The patient had not received dialysis before the COVID-19 outbreak, but her kidney function deteriorated remarkably during hospitalization, leading to the initiation of CVVH. Moreover, infraglottic granulomas from the patient’s GPA complicated intubation and tube removal after tracheostomy, further indicating that the patient’s comorbidities played a significant role in complicating not only the disease course, but also therapeutic choices.
The patient’s immunosuppressive regimen also had to be evaluated. Long-term immunosuppression in transplant recipients is a debatable topic. While some studies report that such treatment increases the risk of acquiring COVID-19, other studies suspect that immunosuppression may disrupt the cytokine storm and thus exert a “protective” effect [2]. In this case, dexamethasone treatment was initiated because of its mortality-reducing effect in COVID-19 patients [12]. Dexamethasone was continued at the time of intubation, although tacrolimus was paused to ease her immunosuppressive load, according to current guidelines. Reduction in immunosuppression is a common intervention in kidney transplant recipients who have COVID-19 [13]. Convalescent plasma therapy also seemed beneficial, although there was no notable association between this treatment and reduced mortality or improved clinical outcomes in non-immunosuppressed patients [14]. Several SARS-CoV-2 monoclonal antibodies are currently available. Regen-Cov (casirivimab and imdevimab) and Xevudy (sotrovimab) are now available under emergency use authorization [15],[16]. Although these agents were not available at the time of hospitalization of our patient, they might be crucial for selected high-risk patients, such as in our case, if one is able to administer them early enough and not at the time of hospitalization.
Venovenous extracorporeal membrane oxygenation also plays an important role in the treatment of patients. However, the use of VV-ECMO for COVID-19-associated ARDS remains controversial. The initial literature reported poor outcomes and uncertainty regarding which patients might benefit the most, long-term outcomes, use of resources, and cost-to-benefit ratio [17]. Subsequently, considerably better outcomes were reported. Venovenous extracorporeal membrane oxygenation is a lifesaving intervention that leads to reduced mortality in selected patients with COVID-19-associated ARDS [18]. However, prolonged pre-ECMO ventilation is associated with increased mortality in COVID-19 patients due to barotrauma, volotrauma, and infections [19]. Thus, early intervention is important, and we believe that VV-ECMO initiation was performed at an ideal time in our patient.
During intubation, the patient was ventilated in prone positioning. Patients with COVID-19-associated ARDS tend to respond well to prone positioning, experience improved oxygenation, and have reduced mortality [20]. We believe that prone positioning was a crucial intervention in our patient.
Interestingly, the patient’s kidney function returned to habitual levels after discharge, even though COVID-19 patients have a higher risk of eGFR decline than those not infected by COVID-19 [21]. This might indicate that the tailoring of immunosuppressive medications was close to optimal. Moreover, fluid management using CVVH also has other benefits besides the management of acute kidney failure. Pulmonary edema due to increased capillary permeability, resulting from acute lung injury, is worsened by a positive fluid balance. Thus, fluid management improves lung function and shortens the duration of both mechanical ventilation and intensive care in patients with acute lung injury [22].
In January 2021, the Norwegian COVID-19 immunization program prioritized individuals who were considered high-risk persons: nursing home residents and elderly people (aged ≥ 85 years). Thus, the patient was not vaccinated at the time of infection. Indeed, vaccination might have been futile because single organ transplant recipients more frequently experience a poor response to COVID-19 vaccination compared to that of the average population, and they are thus more susceptible to post-vaccination breakthrough with SARS-CoV-2 [23]. Nevertheless, it is crucial to develop an intensified vaccination approach to ensure that kidney transplant recipients are effectively vaccinated, as they are vulnerable to COVID-19 [24].
Conclusion
In summary, we described an immunosuppressed kidney transplant recipient who recovered successfully from COVID-19-associated ARDS and multiorgan failure despite several comorbidities that complicated the disease course and therapeutic choices. Treatment with ventilation in a prone position, convalescent plasma, dexamethasone, CVVH, and VV-ECMO was performed in this patient. In view of this case and similar other cases, we have gradually improved treatment approaches for COVID-19 in immunosuppressed patients. Randomized studies and systematization of case reports will further aid in improving patient outcomes.
REFERENCES
1.
Caillard S, Chavarot N, Francois H, et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant 2021;21(3):1295–303. [CrossRef]
[Pubmed]

2.
Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 2020;98(6):1540–8. [CrossRef]
[Pubmed]

3.
Zhu L, Xu X, Ma K, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant 2020;20(7):1859–63. [CrossRef]
[Pubmed]

4.
Arpali E, Akyollu B, Yelken B, Tekin S, Turkmen A, Kocak B. Case report: A kidney transplant patient with mild COVID-19. Transpl Infect Dis 2020;22(4):e13296. [CrossRef]
[Pubmed]

5.
Bussalino E, De Maria A, Russo R, Paoletti E. Immunosuppressive therapy maintenance in a kidney transplant recipient with SARS-CoV-2 pneumonia: A case report. Am J Transplant 2020;20(7):1922–4. [CrossRef]
[Pubmed]

6.
Seminari E, Colaneri M, Sambo M, et al. SARS Cov-2 infection in a renal-transplanted patient: A case report. Am J Transplant 2020;20(7):1882–4. [CrossRef]
[Pubmed]

7.
Chen S, Yin Q, Shi H, et al. A familial cluster, including a kidney transplant recipient, of coronavirus disease 2019 (COVID-19) in Wuhan, China. Am J Transplant 2020;20(7):1869–74. [CrossRef]
[Pubmed]

8.
Wang J, Li X, Cao G, Wu X, Wang Z, Yan T. COVID-19 in a kidney transplant patient. Eur Urol 2020;77(6):769–70. [CrossRef]
[Pubmed]

9.
Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID-19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? Am J Transplant 2020;20(7):1875–8. [CrossRef]
[Pubmed]

10.
Yamada M, Matsumoto E, Thomas CP, et al. Case report: Severe COVID-19 in a kidney transplant recipient without humoral response to SARS-CoV-2 mRNA vaccine series. Transplant Direct 2021;7(9):e743.
[Pubmed]

11.
Tekin S, Özdo?an H, Demir MK, Soultan H, Zafar S. Kidney transplantation and COVID-19: Two case reports. Transplant Proc 2021;53(4):1207–10. [CrossRef]
[Pubmed]

12.
RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021;384(8):693–704. [CrossRef]
[Pubmed]

13.
Devresse A, Belkhir L, Vo B, et al. COVID-19 Infection in kidney transplant recipients: A single-center case series of 22 cases from Belgium. Kidney Med 2020;2(4):459–66. [CrossRef]
[Pubmed]

14.
Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: A systematic review and meta-analysis. JAMA 2021;325(12):1185–95. [CrossRef]
[Pubmed]

15.
Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med 2021;385(23):e81. [CrossRef]
[Pubmed]

16.
Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of the neutralizing SARS-CoV-2 antibody sotrovimab in preventing progression of COVID-19: A randomized clinical trial. medRxiv 2021;2021.11.03.21265533. [CrossRef]

17.
Shekar K, Slutsky AS, Brodie D. ECMO for severe ARDS associated with COVID-19: Now we know we can, but should we? Lancet Respir Med 2020;8(11):1066–8. [CrossRef]
[Pubmed]

18.
Ramanathan K, Shekar K, Ling RR, et al. Extracorporeal membrane oxygenation for COVID-19: A systematic review and meta-analysis. Critical Care 2021;25(1):211. [CrossRef]
[Pubmed]

19.
Kunavarapu C, Yeramaneni S, Melo J, et al. Clinical outcomes of severe COVID-19 patients receiving early VV-ECMO and the impact of pre-ECMO ventilator use. Int J Artif Organs 2021;44(11):861–7. [CrossRef]
[Pubmed]

20.
Shelhamer MC, Wesson PD, Solari IL, et al. Prone positioning in moderate to severe acute respiratory distress syndrome due to COVID-19: A cohort study and analysis of physiology. J Intensive Care Med 2021;36(2):241–52. [CrossRef]
[Pubmed]

21.
Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney outcomes in long COVID. J Am Soc Nephrol 2021;32(11):2851–62. [CrossRef]
[Pubmed]

22.
National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354(24):2564–75. [CrossRef]
[Pubmed]

23.
Qin CX, Moore LW, Anjan S, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation 2021;105(11):e265–6.
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Mariell Rivedal - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Yngvar Lunde Haaskjold - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Anne Berit Guttormsen - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Karsten Midtvedt - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Thomas Knoop - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Mariell Rivedal et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.