|
Case Series
Adjusting background insulin therapy in type 2 diabetes when initiating a glucagon-like peptide 1 receptor agonist: A case series
1 Clinical Professor, Department of Pharmacy Practice, Auburn University Harrison College of Pharmacy; Auburn, Alabama, USA
2 Clinical Pharmacy Specialist, Baptist Family Medicine, Baptist Health System; Montgomery, Alabama, USA
3 Doctor of Philosophy Student, Department of Drug Discovery and Development, Auburn University Harrison College of Pharmacy; Auburn, Alabama, USA
Address correspondence to:
Heather P Whitley
4371 Narrow Lane Rd, Suite #100, Montgomery, Alabama 36116,
USA
Message to Corresponding Author
Article ID: 100073Z09HW2023
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Whitley HP, Smith WD. Adjusting background insulin therapy in type 2 diabetes when initiating a glucagon-like peptide 1 receptor agonist: A case series. J Case Rep Images Med 2023;9(1):4–10.ABSTRACT
Introduction: Guidelines recommend preferential use of antihyperglycemic medications with proven cardiovascular benefit for the treatment of patients with type 2 diabetes with established atherosclerotic cardiovascular disease (ASCVD), high risk factors for ASCVD, kidney disease, or heart failure. However, current guidelines offer little to no practical recommendations for adding these therapies to a patient’s current regimen while avoiding hyperglycemia or hypoglycemia. Nevertheless, considering background therapy in a proactive effort to avoid undesirable glycemic excursions when initiating any new antidiabetic medication remains paramount.
Case Series: A six-patient case series investigates adjustments to background therapies and glycemic outcomes surrounding the initiation and titration of long-acting glucagon-like peptide 1 receptor agonists (GLP-1 RAs) to shed light on practical methods to manage patient care during this tenuous phase. Overarching findings regarding background therapy adjustments to avoid hypoglycemia when initiating a GLP-1 RA include: (1) safe continuation of metformin regardless of baseline A1C or concurrent glycemic background therapy; (2) continuation of background therapy when the baseline A1C is above 9%; (3) consideration of a proactive 15–20% basal insulin dose reduction when the baseline A1C is below 7.5%; (4) proactive bolus insulin dose reduction by 25% or complete discontinuation at the time of GLP-1 RA initiation.
Conclusion: No dose adjustments are necessary when A1C > 9%, and possibly >8%. When A1C is <7.5% and possibly <8%, discontinue or reduce bolus insulin by 25% and/or reduce basal insulin by 15–25%. Adjust background therapy using shared-decision making while considering fasting blood glucose, A1C, hypoglycemia risk, and chosen GLP-1 RA therapy.
Keywords: Diabetes mellitus, GLP-1 receptor agonists, Glucagon-like peptide 1 receptor agonists, Hypoglycemia, Insulin, Type 2
Introduction
A small but significant modification was made to the 2020 American Diabetes Association (ADA) Standards of Medical Care in Diabetes [1]. This change highlighted the preferential use of antihyperglycemic medications with proven cardiovascular (CV) benefit for the treatment of type 2 diabetes (T2D) in the presence of established atherosclerotic cardiovascular disease (ASCVD), indicators of high risk for ASCVD, established kidney disease, or heart failure regardless of glycemic control. As such, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and/or sodium-glucose cotransporter 2 (SGLT2) inhibitors are now recommended second-line to metformin and lifestyle modifications regardless when any one of these conditions occurs concomitantly with T2D. This shift in treatment recommendation is now mirrored in the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) Consensus Statement for Comprehensive T2D Management [2] as well as the American College of Cardiology (ACC) 2020 Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients with T2D [3]. Finally, these recommendations have been carried forward into the ADA Standards of Medical Care in Diabetes in 2021 [4] and 2022 [5].
The status of a patient’s glycemic profile remains important to overall clinical management, especially regarding patient safety. Presently, however, practice guidelines offer no practical recommendations for adding these therapies, particularly when a patient’s glycemic status is near, at, or below goal on background therapy. Nevertheless, practitioners considering patient background therapy in a proactive effort to avoid supratherapeutic blood glucose reduction and/or hypoglycemia when initiating any new antidiabetic medication remains paramount. Here, we provide a case series of multiple unique patients, each during their initiation and titration phases of GLP-1 RA therapy. We attributed special attention to adjustments in background antidiabetic medications and glycemic control status to shed light on practical methods to manage glycemic control during this tenuous phase of patient care.
Case Report
Methods
Electronic and paper medical records of T2D patients from a single outpatient family medicine clinic were retrospectively reviewed. Patients were reviewed if a GLP-1 RA was initiated between October 2016 and January 2020. Cases were selected to demonstrate changes to glycemic status following GLP-1 RA initiation and adjustments to background therapy. Accordingly, cases were excluded when surrounding factors interfered, such as early loss to follow-up or early GLP-1 RA discontinuation. GLP-1 RA product, timing of initiation, and dose titrations were paired to blood glucose status, and changes to background antidiabetic therapies.
Case 1
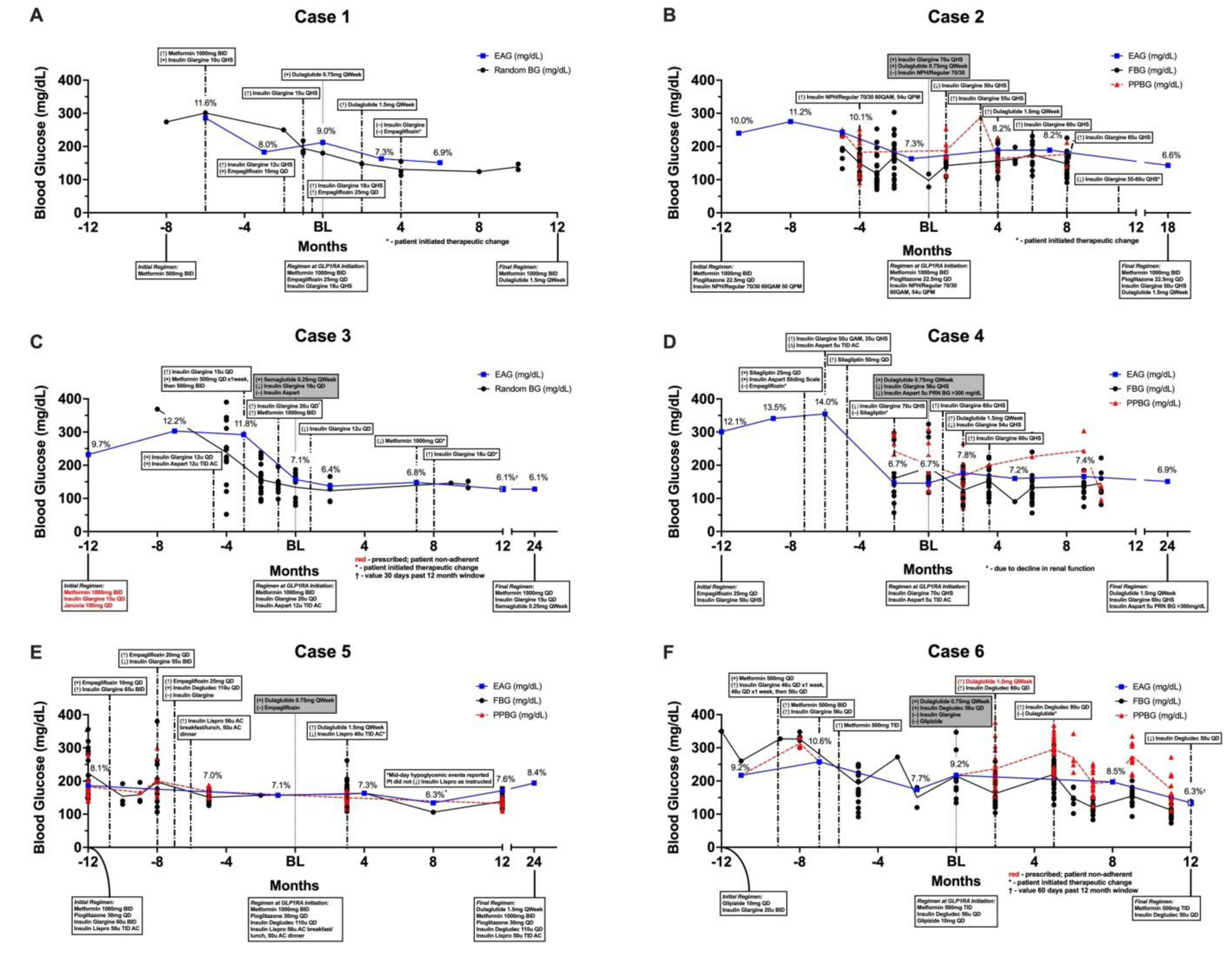
Case 1 was a 48-year-old Caucasian male with a 20-month history of T2D. At the time of GLP-1 RA initiation, he was using metformin 1000 mg by mouth twice daily, empagliflozin 25 mg by mouth once daily, and insulin glargine 18 units subcutaneously at bedtime. His A1C was 9.0% at the time of GLP-1 RA initiation, having declined from 11.6% six months earlier. Dulaglutide was initiated at the lowest dose of 0.75 mg subcutaneously once weekly and subsequently titrated to 1.5 mg two months later. No adjustments to antihyperglycemic background therapy were made until month four of GLP-1 RA use when the patient self-discontinued insulin glargine and empagliflozin after his A1C declined to 6.9% (Figure 1A).
Case 2
Case 2 was a 71-year-old Caucasian male with a 23-year history of T2D. At the time of GLP-1 RA initiation, he used metformin 1000 mg by mouth twice daily, pioglitazone 45 mg one-half tablet by mouth once daily, and mixed insulin NPH/regular 70/30 60 units before breakfast and 54 units before dinner. His A1C was 7.3% at the time of GLP-1 RA initiation, having declined from 10.1% four months earlier. Dulaglutide was initiated at the lowest dose of 0.75 mg subcutaneously once weekly along with insulin glargine (Basaglar) 70 units subcutaneously at bedtime. Insulin NPH/regular 70/30 was discontinued while metformin and pioglitazone were continued. One month later, the insulin glargine dose was decreased to 50 units (29% decrease) due to the patient’s report of hypoglycemia. Dulaglutide was titrated to 1.5 mg subcutaneously once weekly after four months of use at the lower dose, and insulin glargine was again adjusted upward to 55 units (10% increase). Since that time, he has continued using metformin, pioglitazone, and dulaglutide 1.5 mg with small alterations to his dose of insulin glargine (55–65 units daily). Current plans are to increase his dulaglutide dose to 3 mg to allow for further reductions in his basal insulin therapy. His most recent A1C was 6.6% (Figure 1B).
Case 3
Case 3 was a 59-year-old Black male with a 14-year history of T2D, cerebral vascular accident (CVA), and other comorbid diseases. At the time of GLP-1 RA initiation, his A1C was 7.1% using metformin 500 mg by mouth once daily, insulin glargine U300 20 units subcutaneously at bedtime, and insulin aspart 12 units subcutaneously three times daily with meals. When semaglutide 0.25 mg subcutaneously once weekly was initiated, glargine was reduced from 20 to 16 units (20% decrease) and insulin aspart was discontinued. Although semaglutide was not further titrated, his basal insulin dose was decreased to 12 units daily (25% decrease) and metformin dose decreased to 1000 mg by mouth once daily (50% decrease). His A1C was maintained below goal for twenty-four months following GLP-1 RA initiation (Figure 1C).
Case 4
Case 4 was a 68-year-old Caucasian female with a 16-year history of T2D. A year before GLP-1 RA initiation her A1C was 12.1%. Upon GLP-1 RA initiation her A1C was 6.7% and her regimen included insulin glargine 70 units subcutaneously at bedtime and insulin aspart 5 units subcutaneously three times daily before meals. To offset the risk of hypoglycemia when dulaglutide 0.75 mg subcutaneously once weekly was initiated, insulin glargine was reduced from 70 to 56 units subcutaneously at bedtime (20% decrease) and insulin aspart was removed from scheduled to as needed when blood glucose (BG) > 300 mg/dL. Insulin glargine was increased to 60 units (7% increase) after one month, but again reduced to 54 units alongside up-titration of dulaglutide to 1.5 mg in the second month of therapy. Insulin glargine was increased back to 60 units at three months. These collective adjustments resulted in an A1C of 7.2% around six months after GLP-1 RA initiation (Figure 1D).
Case 5
Case 5 was a 39-year-old Black female with a 24-year history of T2D using insulin degludec U200 100 units subcutaneously daily, insulin lispro 56 units subcutaneously before breakfast/lunch and 50 units before dinner, metformin 1000 mg by mouth twice daily, pioglitazone 30 mg by mouth once daily, and empagliflozin 25 mg by mouth once daily with a resulting A1C of 7.1%. Multiple genitourinary infections, attributed to the SGLT2 inhibitor, prompted discontinuation of empagliflozin and initiation of dulaglutide 0.75 mg subcutaneously once weekly. All other therapies were continued at their respective doses. Four months later her A1C was 7.3%, and her dulaglutide dose was increased to 1.5 mg subcutaneously once weekly with a recommended reduction in her pre-meal insulin lispro dose to 40 units (~25% decrease). For unknown reasons, the patient did not make the instructed change to her bolus insulin dose, with a resulting A1C of 6.3% that was likely heavily influenced by hypoglycemic events that occurred mid-day. One year after discontinuation of empagliflozin and initiation of dulaglutide, her A1C was 7.6% (Figure 1E).
Case 6
Case 6 was an 82-year-old Caucasian male with a 36-year history of T2D. Dulaglutide 0.75 mg subcutaneously once weekly was started in response to his increased A1C of 9.2%. Glipizide was discontinued and insulin degludec was substituted for insulin glargine given his advanced age and importance of avoiding hypoglycemia. Metformin 500 mg three times daily was continued without change. The patient was prescribed dulaglutide 1.5 mg five weeks later, but never filled the prescription due to cost. Instead, he completed five months of dulaglutide 0.75 mg and then self-discontinued GLP-1 RA therapy. After that point, his self-monitored blood glucose (SMBG) became more erratic, requiring basal insulin intensification. Bolus insulin was never initiated due to his age and inability to manage a complicated regimen (Figure 1F).
Discussion
In accordance with current guideline recommendations, antidiabetic medication regimens for patients with T2D and established ASCVD or high ASCVD risk factors should include an agent with proven benefit to reduce MACE irrespective of glycemic status [4]. Within the GLP-1 RA class, those with proven cardiovascular benefit are liraglutide, dulaglutide, and subcutaneous semaglutide [6],[7],[8]. Glucagon-like peptide 1 receptor agonists do not inherently increase the risk for hypoglycemia, but this risk is augmented when GLP-1 RAs are added to therapies known for causing hypoglycemia (i.e., sulfonylureas or insulin) [9].
With the exception of an elderly patient using a sulfonylurea (Case 6), patients in this case series with baseline A1C > 9% needed no proactive adjustments to background therapy when a GLP-1 RA was started. Conversely, proactive adjustments to background therapy were prudent for patients with baseline A1C < 7.5% using insulin. In Cases 2, 3, and 6, the basal insulin dose was initially reduced by 13–20%. While the initial 20% basal insulin dose reduction for Case 3 was adequate at the time of GLP-1 RA initiation, a subsequent 25% reduction was later required to avoid hypoglycemia. A proactive 20% basal insulin dose reduction was used in Case 4, but a slight basal dose increase was implemented one month later for an overall dose adjustment of 14% from baseline. In Case 2, insulin therapy was simultaneously switched and reduced by 13% with a change from pre-mixed insulin to basal-only. However, this patient still required a subsequent dose decrease one month following GLP-1 RA initiation for an overall 38% reduction. This dose reduction was seemingly too aggressive given Case 2’s A1C did not decline closer to goal until the GLP-1 RA and basal insulin were later titrated. Interestingly, Case 5 did not need a basal dose adjustment despite a baseline A1C of 7.1% since the GLP-1 RA was substituted for a SGLT2 inhibitor. At least in this case, the starting dose of dulaglutide is equally effective in terms of glycemic control to empagliflozin 25 mg. Subsequent insulin adjustments were not needed until the GLP-1 RA was titrated, at which time a 26% bolus insulin dose reduction was recommended but not followed. The patient later experienced midday hypoglycemia, which reinforced the need to decrease the bolus insulin dose. Meanwhile, bolus insulin therapy was entirely discontinued (Cases 2 and 3) or moved from scheduled to as-needed (Case 4) at the time of GLP-1 RA initiation. In all cases where renal function allowed, metformin was maintained throughout the course of therapy. Case 3 remains a slight exception given the self-reduced from twice-daily to once-daily administration for unknown reasons. This patient-initiated therapeutic change likely necessitated the increase in basal insulin dose eight months after GLP-1 RA initiation.
Several practical implications can be gleaned from this case series. Firstly, adjustments to background insulin therapy are not necessary when baseline A1C is above 9% but should be considered when the A1C is below 7.5%. This observation is supported by several phase 3 clinical trials, which describe preemptive therapeutic adjustments within their study protocols aimed to reduce the risk of hypoglycemia when a long-acting GLP-1 RA is added to basal insulin therapy. Most of these trials implemented an initial 20% insulin dose reduction at the time of GLP-1 RA initiation in patients with baseline A1C = 8% [10],[11]. In yet another large-scale investigation that recommended proactive adjustments to background therapy in individuals with baseline A1C = 7%, basal insulin doses were reduced by 15%, total daily doses of sulfonylurea therapy were reduced by one dose level, and mealtime meglitinide was either discontinued or reduced by one dose level [6]. While these studies are not entirely congruent in their recommendation for adjusting background basal insulin, this case series highlights that an empiric basal insulin dose reduction between 15% and 20% upon GLP-1 RA initiation is reasonable and prudent in most patients with an A1C of =7.5% to avoid hypoglycemia. Secondly, bolus insulin may be safely reduced by 25%, or altogether discontinued in some patients, along with a 15–25% reduction in basal insulin dose at the time of GLP-1 RA initiation. This is supported by a study demonstrating that the addition of a long-acting GLP-1 RA to basal-bolus insulin therapy allowed for the discontinuation of bolus insulin in half of study participants while improving glycemic control. In this study, mealtime insulin was initially reduced by 50% at initiation of the long-acting GLP-1 RA [12]. After initial bolus insulin dose reduction, prandial insulin can be discontinued, reinitiated, or titrated as needed for glycemic control within an individual patient. Lastly, metformin can be safely continued upon GLP-1 RA initiation regardless of baseline A1C or concurrent glycemic background therapy.
While this case study provides insight as to the logistics of safely adding a GLP-1 RA to insulin-based therapeutic regimens, there are some noteworthy limitations. This retrospective assessment was not controlled, and individual patients were not fully assessed for behavioral modifications that potentially impacted therapeutic outcomes. Though all patients completed diabetes self-management education prior to GLP-1 RA initiation, adjustments to diet or physical activity leading to observed changes in blood glucose cannot be ruled out. Likewise, adherence to medications was not monitored objectively. Patients were systematically assessed for adherence issues, BG testing frequency, and self-monitored blood glucose (SMBG) values at every visit. However, all of these parameters are completely reliant on subjective input. Two patients self-modified their therapy; one reduced the dose of metformin for unelaborated reasons and another discontinued GLP-1 RA therapy due to cost. This speaks to the importance of verifying a patient’s ability to access and afford all prescribed medications. This study did not provide guidance for non-insulin-based therapy except for metformin, which was used by all but the patient in Case 4. Insight for insulin adjustments needed in patients with baseline A1C between 7.5% and 9% was also not provided. However, one may assume that a less aggressive insulin dose reduction may be needed compared to those with lower A1Cs. Only long-acting dulaglutide and semaglutide were evaluated through this case series; oral semaglutide had not yet been FDA approved [13]. Lastly, this case series occurred before FDA approval of higher doses of dulaglutide [14] and semaglutide [15].
Some patients would predictably achieve greater benefits through further titrating their GLP-1 RA, which would allow for greater insulin dose reductions within their regimens.
Conclusion
Glucagon-like peptide 1 receptor agonists can be safely added to insulin-based background therapeutic regimens. No dose adjustments are likely needed when A1C > 9%, and possibly >8%. Follow-up may safely occur one to two months after GLP-1 RA initiation without great concern for risk of hypoglycemia, although it may be sensible to follow-up earlier (2–4 weeks) for other reasons including proper GLP-1 RA administration and tolerance. Alternatively, when A1C is <7.5% and possibly <8%, a 25% bolus insulin dose reduction or complete discontinuation should be considered alongside a 15–25% basal insulin dose reduction to mitigate risk of hypoglycemia. In these situations, it remains prudent to follow-up at one month to reevaluate glycemic status considering therapeutic adjustments. Still, without firm evidence guiding logistical implementation of GLP-1 RA in patients with near-controlled blood glucose and using insulin-based therapy, background therapy adjustments should be made using shared-decision making on a case-by-case basis while considering factors such as fasting blood glucose, current A1C, hypoglycemia risk, specific GLP-1 RA therapy, and GLP-1 RA therapy titration schedule.
REFERENCES
1.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes—2020. Diabetes Care 2020;43(Suppl 1):S98–110. [CrossRef]
[Pubmed]

2.
Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American association of clinical endocrinologists and American college of endocrinology on the comprehensive type 2 diabetes management algorithm – 2020 executive summary. Endocr Pract 2020;26(1):107–39. [CrossRef]
[Pubmed]

3.
Das SR, Everett BM, Birtcher KK, et al. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: A report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol 2020;76(9):1117–45. [CrossRef]
[Pubmed]

4.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes—2021. Diabetes Care 2021;44(Suppl 1):S111–24. [CrossRef]
[Pubmed]

5.
American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes—2022. Diabetes Care 2022;45(Suppl 1):S125–43. [CrossRef]
[Pubmed]

6.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019;394(10193):121–30. [CrossRef]
[Pubmed]

7.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375(19):1834–44. [CrossRef]
[Pubmed]

8.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375(4):311–22. [CrossRef]
[Pubmed]

9.
Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020;98(4S):S1–115. [CrossRef]
[Pubmed]

10.
Pozzilli P, Norwood P, Jódar E, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9). Diabetes Obes Metab 2017;19(7):1024–31. [CrossRef]
[Pubmed]

11.
Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): A randomized, controlled trial. J Clin Endocrinol Metab 2018;103(6):2291–301. [CrossRef]
[Pubmed]

12.
Rosenstock J, Nino A, Soffer J, et al. Impact of a weekly glucagon-like peptide 1 receptor agonist, albiglutide, on glycemic control and on reducing prandial insulin use in type 2 diabetes inadequately controlled on multiple insulin therapy: A randomized trial. Diabetes Care 2020;43(10):2509–18. [CrossRef]
[Pubmed]

13.
Rybelsus (semaglutide tablets) [product information]. Plainsboro, NJ: Novo Nordisk Inc, 2023. [Available at: https://www.rybelsus.com/prescribing-information.html?&utm_source=google&utm_medium=cpc&utm_term=rybelsus&utm_campaign=&utm_content=_-mkwid-s_dc-pcrid-587115119930-pkw-rybelsuspmt-e-&gclid=CjwKCAjw9J2iBhBPEiwAErwpeeU9iqmgmfyLNyVLvJUqGwd4RRDVVvd8Mp5Sn0LxlRiwyqsmC3QShoCQR4QAvD_BwE&gclsrc=aw.ds]

14.
Trulicity (dulaglutide) injection [product information]. Indianapolis, IN: Eli Lilly and Co, 2020. [Available at: https://uspl.lilly.com/trulicity/trulicity.html#pi]

15.
Ozempic (semaglutide injection) [product information]. Plainsboro, NJ: Novo Nordisk Inc, 2022. [Available at: https://www.novo-pi.com/ozempic.pdf]

SUPPORTING INFORMATION
Author Contributions
Heather P Whitley - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Warren D Smith - Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2023 Heather P Whitley et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.