|
Case Report
Cement pulmonary embolism as a complication of percutaneous vertebroplasty in a patient with follicular lymphoma
1 MD, Department of Internal Medicine, Centro Hospitalar Universitário de Coimbra, Coimbra, Portugal
Address correspondence to:
Mariana Guerra
Quinta dos Vales, Coimbra,
Portugal
Message to Corresponding Author
Article ID: 100074Z09MG2023
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Guerra M, Peixoto J, Marado D. Cement pulmonary embolism as a complication of percutaneous vertebroplasty in a patient with follicular lymphoma. J Case Rep Images Med 2023;9(1):11–15.ABSTRACT
Vertebroplasty involves injecting a cement polymer, often polymethylmethacrylate (PMA), into the vertebral body under imaging guidance to increase stability. However, the high vascularization and anatomical network of the paravertebral and extradural venous plexuses can allow the migration of cement particles into the systemic venous circulation regardless of whether spinal compression or fractures occur during or are present prior to the treatment. This case report presents a 42-year-old female patient who visited the emergency room with symptoms of cough, nasal obstruction, rhinorrhea, and dyspnea and had a history of follicular non-Hodgkin’s lymphoma under rituximab treatment. Imaging revealed a cement embolism in the pulmonary artery tree, most likely caused by prior vertebroplasty. Anticoagulation was started despite the lack of hypoxemia due to the inorganic character of the embolic substance and the patient’s immunosuppressed status. The embolic debris was still present on subsequent imaging, but the patient’s condition remained stable, with some signs of illness remission. This case highlights the importance of considering cement embolism as a possible vertebroplasty complication and the importance of properly assessing and managing such cases, particularly in patients with underlying medical issues, as well as the need for the development of a standard protocol of sequential chest X-rays after the procedure and possible alternatives to PMA.
Keywords: Cement pulmonary embolism, Metastatic compression fractures, Procedure complications, Vertebroplasty
Introduction
Percutaneous vertebroplasty is a minimally invasive surgical procedure guided by imaging utilized for managing painful tumor infiltration disorders such as aggressive hemangioma, metastatic carcinoma, and multiple myeloma. Additionally, it can be employed for individuals experiencing severe pain resulting from osteoporotic thoracolumbar fractures. Despite its effectiveness in treating vertebral compression fractures, percutaneous vertebroplasty is not without risks. Complications that may arise from this procedure include allergic reactions, infections, neurologic complications, and cement leakage into the vertebrae and surrounding tissues, which may result in nerve damage or other complications. In rare cases, pulmonary embolism can occur when bone cement enters the bloodstream and causes lung blockage. The entity of pulmonary cement embolisms is in most cases asymptomatic and occurs in up to 26% after vertebroplasty [1].
Case Report
We present the case of a 42-year-old female patient who went to the emergency department (ER) for a 1-month history of productive cough, nasal obstruction, rhinorrhea, and dyspnea on moderate exertion.
She denied fever, gastrointestinal, or genitourinary symptoms. The patient was diagnosed about five years ago with follicular non-Hodgkin’s lymphoma, currently in stage IV, and recently completed the tenth maintenance cycle with Rituximab (a dosage of 600 mg administered every eight weeks). Due to persistent lumbar pain refractory to analgesics and anti-inflammatory drugs two years ago, a computed tomography (CT) scan of the lumbar spine was performed, which revealed a fracture with compression of L1. A complementary investigation with magnetic resonance imaging and a last positron emission tomography (PET) scan revealed multiple bone lesions in the sternal notch, costal arches, humerus, shoulder blades, and axial skeleton. These were suggestive of multiple metastatic lesions. The patient underwent vertebroplasty with a bone biopsy and the immunohistopathological examination confirmed the diagnosis of a metastatic lesion secondary to follicular non-Hodgkin’s lymphoma. The procedure was uneventful, but no thoracic imaging was performed afterward.
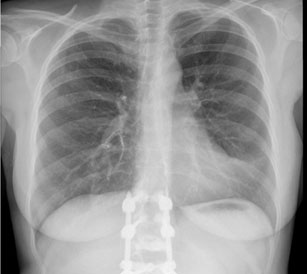
When admitted to the ER, the patient was apyretic and hemodynamically stable, with a peripheral saturation of 98% in room air. The partial pressure of oxygen in arterial blood was 102.1 mmHg. The SARS-CoV-2 screening swab was negative. The complementary study showed D-dimers at 0.29 mg/mL, high-sensitivity troponin at 1.9 ng/mL, and C-reactive protein at 1.43 mg/dL. The chest X-ray showed a well-defined tubular image at the para-direct hilar (Figure 1).
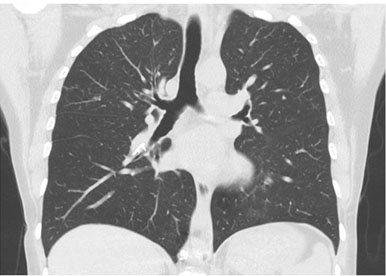
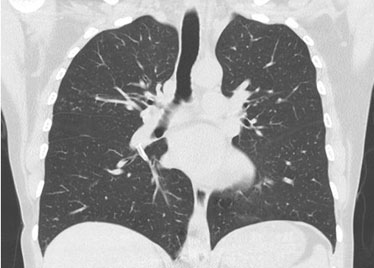
A thoracic computed tomography (CT) scan showed osteofixation material in the lumbar spine and high-density material in the shape of a line in the lumen of the pulmonary artery tree on both sides, at the level of the azygos and hemiazygos veins and in the inferior vena cava. The material stood out at the level of the inferior lobar artery of the right lung, with extension to the posterior and medial basal segmental branches. In the left lung, the material was identified in the interlobar artery and the superior segmental branch (Figure 2 and Figure 3). Those aspects were associated with cement embolism in the context of previous vertebroplasty. Further evaluation ruled out other embolic foci and deep vein thrombosis in the lower limbs. There were no indications of right ventricular dysfunction seen during the transthoracic echocardiography.
Even though the patient did not have hypoxemia, it was decided to start anticoagulation with enoxaparin (1 mg/kg intravenously every 12 hours) for six months due to the inorganic nature of the substance, the patient’s state of immunosuppression and the central and peripheral involvement of cement embolism sites. Three months after her ER visit, a chest CT scan confirmed that the embolic debris remained present. The patient remained with no symptoms and maintained partial disease remission throughout the eleventh cycle of Rituximab.
Discussion
A vertebral compression fracture (VCF) is a significant contributor to spinal pain which can be attributed to osteoporosis and primary or metastatic cancer. The available treatment for VCF comprises conservative measures such as bed rest, analgesics, and back braces, as well as more invasive interventions like percutaneous vertebroplasty (PVP) and open surgery [2]. Due to the prevalence of osteoporosis risk factors, such as advanced age and corticosteroid treatment, cancer patients are at high risk for these fractures [3]. In the case presented, the patient had a history of follicular non-Hodgkin’s lymphoma with multiple bone metastases and had previously undergone vertebroplasty for a vertebral compression fracture using polymethylmethacrylate (PMA) bone cement.
Percutaneous vertebroplasty is a minimally invasive procedure involving the injection of polymethylmethacrylate (PMA) bone cement into the vertebral body. Polymethylmethacrylate leakage is the most common cause of complications following these procedures, and it typically occurs when the cement is too fluid or when too much pressure is applied [4]. Polymethylmethacrylate can sometimes penetrate the venous plexuses or, by retrograde migration, enter the aorta [5], causing pulmonary cement embolism (PCE). Its precise prevalence is unknown, ranging from 2% to 26%, depending on the diagnostic imaging technique chosen [1]. Pulmonary cement embolism often has no noticeable symptoms and manifests within days to weeks after the treatment, or sometimes immediately. The patient didn’t report any symptoms after the procedure. Nevertheless, the patient’s neoplastic condition may have underestimated the symptoms. These estimates are likely underestimated due to the expected absence of symptoms and the use of protocols that do not include thoracic imaging testing after the procedure [6].
There is no standardized treatment for PCE, and the present data are mainly based on clinical cases [7]. Bone cement (BC) does not appear to be thrombogenic or to stimulate coagulation, but its turbulent flow may enhance prothrombotic processes and vascular injury [8]. In 2009, Krueger et al. published a comprehensive review recommending being vigilant in peripheral and asymptomatic embolisms and starting anticoagulation with heparin for three to six months, followed by warfarin cases of central and symptomatic embolisms. The existence of symptoms, as well as the location and size of embolic particles, now determine the treatment strategy. Surgical embolectomy may be considered for severe and symptomatic embolisms.
It is unclear if the prothrombotic nature of cancer would affect the risk of PCE or the therapy method, such as in peripheral and silent embolisms. Low-molecular-weight heparin therapy for central and symptomatic embolisms may be combined with chemotherapy or immunotherapy. In the presented case, despite the patient being asymptomatic, the decision was made to initiate anticoagulation with enoxaparin due to the central and peripheral involvement of the cement embolism sites, the patient’s immunosuppressed state, and the inorganic nature of the cement. The follow-up chest computed tomography (CT) scan performed three months later confirmed the presence of embolic debris. Although there are now seven related fatalities described in the literature [9],[10],[11],[12],[13],[14],[15], PCE has a primarily favorable prognosis.
The risk of embolization may be decreased by employing techniques like prone positioning to reduce intrathoracic pressure; venography with fluoroscopy before the cement injection to detect leaks; a standard protocol of sequential chest X-rays after the procedure; and yearly and BC substitutes [16].
Conclusion
This case report highlights the importance of considering PCE as a potential complication in patients undergoing vertebroplasty, particularly in high-risk patients and emphasizes vigilance in detecting and managing PCE, as it can be asymptomatic or underreported. Many topics, such as PCE’s natural history and the most effective preventative measures, still need to be answered. There needs to be a standardized treatment for PCE, which is now individualized based on the presence and severity of symptoms. With this case, we aim to raise awareness for the possibility of PCE following vertebroplasty and the importance of early detection and appropriate management.
REFERENCES
1.
McCall T, Cole C, Dailey A. Vertebroplasty and kyphoplasty: A comparative review of efficacy and adverse events. Curr Rev Musculoskelet Med 2008;1(1):17–23. [CrossRef]
[Pubmed]

2.
Gangi A, Sabharwal T, Irani FG, et al. Quality assurance guidelines for percutaneous vertebroplasty. Cardiovasc Intervent Radiol 2006;29(2):173–8. [CrossRef]
[Pubmed]

3.
Mansour A, Abdel-Razeq N, Abuali H, et al. Cement pulmonary embolism as a complication of percutaneous vertebroplasty in cancer patients. Cancer Imaging 2018;18(1):5. [CrossRef]
[Pubmed]

4.
Krueger A, Bliemel C, Zettl R, Ruchholtz S. Management of pulmonary cement embolism after percutaneous vertebroplasty and kyphoplasty: A systematic review of the literature. Eur Spine J 2009;18(9):1257–65. [CrossRef]
[Pubmed]

5.
Matouk CC, Krings T, Ter Brugge KG, Smith R. Cement embolization of a segmental artery after percutaneous vertebroplasty: A potentially catastrophic vascular complication. Interv Neuroradiol 2012;18(3):358–62. [CrossRef]
[Pubmed]

6.
Geraci G, Lo Iacono G, Lo Nigro C, Cannizzaro F, Cajozzo M, Modica G. Asymptomatic bone cement pulmonary embolism after vertebroplasty: Case report and literature review. Case Rep Surg 2013;2013:591432. [CrossRef]
[Pubmed]

7.
Kim YJ, Lee JW, Park KW, et al. Pulmonary cement embolism after percutaneous vertebroplasty in osteoporotic vertebral compression fractures: Incidence, characteristics, and risk factors. Radiology 2009;251(1):250–9. [CrossRef]
[Pubmed]

8.
Kollmann D, Hoetzenecker K, Prosch H, et al. Removal of a large cement embolus from the right pulmonary artery 4 years after kyphoplasty: Consideration of thrombogenicity. J Thorac Cardiovasc Surg 2012;143(4):e22–4. [CrossRef]
[Pubmed]

9.
Chen HL, Wong CS, Ho ST, Chang FL, Hsu CH, Wu CT. A lethal pulmonary embolism during percutaneous vertebroplasty. Anesth Analg 2002;95(4):1060–2. [CrossRef]
[Pubmed]

10.
Stricker K, Orler R, Yen K, Takala J, Luginbühl M. Severe hypercapnia due to pulmonary embolism of polymethylmethacrylate during vertebroplasty. Anesth Analg 2004;98(4):1184–6. [CrossRef]
[Pubmed]

11.
Monticelli F, Meyer HJ, Tutsch-Bauer E. Fatal pulmonary cement embolism following percutaneous vertebroplasty (PVP). Forensic Sci Int 2005;149(1):35–8. [CrossRef]
[Pubmed]

12.
Marden FA, Putman CM. Cement-embolic stroke associated with vertebroplasty. AJNR Am J Neuroradiol 2008;29(10):1986–8. [CrossRef]
[Pubmed]

13.
D’Errico S, Niballi S, Bonuccelli D. Fatal cardiac perforation and pulmonary embolism of leaked cement after percutaneous vertebroplasty. J Forensic Leg Med 2019;63:48–51. [CrossRef]
[Pubmed]

14.
Razuin R, Effat O, Shahidan MN, Shama DV, Miswan MFM. Bone cement implantation syndrome. Malays J Pathol 2013;35(1):87–90.
[Pubmed]

15.
Yoo KY, Jeong SW, Yoon W, Lee J. Acute respiratory distress syndrome associated with pulmonary cement embolism following percutaneous vertebroplasty with polymethylmethacrylate. Spine (Phila Pa 1976) 2004;29(14):E294–7. [CrossRef]
[Pubmed]

16.
McGraw JK, Heatwole EV, Strnad BT, Silber JS, Patzilk SB, Boorstein JM. Predictive value of intraosseous venography before percutaneous vertebroplasty. J Vasc Interv Radiol 2002;13(2 Pt 1):149–53. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Mariana Guerra - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
João Peixoto - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Daniela Marado - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2023 Mariana Guerra et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.